For a simple publication list (not as much fun), click here
patents
1. “Solid State Ion Conduction in ZnPS3,”
Kimberly A. Robb (aka See), Andrew J. Martinolich, US Patent No.: 011749825 B2, Sept. 5, 2023.
book chapters
1. “Battery materials”
Zachery W. B. Iton, Seong Shik Kim, Eshaan S. Patheria, Michelle D. Qian, Skyler D. Ware, and Kimberly A. See,
Comprehensive Inorganic Chemistry III 2023, 308-363.
https://doi.org/10.1016/B978-0-12-823144-9.00110-2
publications
60. “The Influence of Metal and Anion Electronic States on the Stability of High Valent Metals”
Colin T. Morrell, Victoria K. Davis, Nicholas V. Dulock, Eshaan S. Patheria, Kimberly A. See*.
Coord. Chem. Rev. 2025, accepted.
tl;dr Coming soon!

59. “The Dynamical Role of Optical Phonons and Sublattice Screening in a Solid-State Ion Conductor”
J. Am. Chem. Soc. 2025, 147, 26456-26467.
https://doi.org/10.1021/jacs.5c06064
58. “On the Structural Origin of Fast Li-Ion Cycling in Tetragonal Bronze-Type Nb8W9O47”
Chem. Mater. 2025, 37, 5158-5166.
https://doi.org/10.1021/acs.chemmater.5c00827
57. “Classification of (Dis)ordered Structures as Superionic Lithium Conductors with an Experimental Structure-Conductivity Database”
Daniel B. McHaffie, Zachery W. B. Iton, Jadon M. Bienz, Forrest A. L. Laskowski and Kimberly A. See*.
Digital Discovery 2025, accepted.
https://dx.doi.org/10.1039/D5DD00052A
tl;dr Data-driven discovery of superionic solid-state electrolytes (SSEs) has been hindered by the lack of conductivity-labeled structural data and features that can represent disorder. We curate the largest such dataset to date and show that graph-based features enable learning directly from disordered crystals. AtomSets models trained on these features identify 241 candidate Li superionic conductors from the ICSD and Materials Project. One top candidate, Li₉B₁₉S₃₃, is experimentally validated and shown to exhibit superionic conductivity.

56. “Cooperative Effects Associated with High Electrolyte Concentrations in Driving the Conversion of CO2 to C2H4 on Copper”
Shaoyang Lin, Yuval Fishler, Soonho Kwon, Annette E. Böhme, Weixuan Nie, Matthias H. Richter, Moon Young Yang, Jesse E. Matthews, Zachery W. B. Iton, Brian C. Lee, Thomas F. Jaramillo, Harry A. Atwater, William A. Goddard III*, Wilson A. Smith*, Kimberly A. See*. [ChemRxiv]
Chem Cat. 2025, accepted.
https://doi.org/10.1016/j.checat.2025.101338

tl;dr We explore the effect of increasing supporting electrolyte concentration on the product profiles associated with electrochemical CO2 reduction. Higher electrolyte concentrations favor C2 products over C1 products. Though it would be satisfying to correlate the shift in product distribution to a change in a singular property, we observe that several properties are correlated to the change in product distribution. Bulk electrolyte properties are changing as electrolyte concentration increases, but the processes in the double layer also change. The binding mode of intermediates is even affected by electrolyte concentration! Thus, we cannot draw a single conclusion as to the mechanism by which the product profile has shifted.
55. “High Energy Density Li-Ion Battery Cathode Using Only Industrial Elements,”
Eshaan S. Patheria, Pedro Guzman, Leah S. Soldner, Michelle D. Qian, Colin T. Morrell, Seong Shik Kim, Kyle Hunady, Elena R. Priesen Reis, Nicholas V. Dullock, James R. Neilson, Johanna Nelson Weker, Brent Fultz, Kimberly A. See*
J. Am. Chem. Soc. 2025, 147, 9786-9799.
https://doi.org/10.1021/jacs.4c18440
tl;dr Substitution of Al into Li2FeS2 stabilizes the material upon electrochemical oxidation resulting in higher gravimetric capacities and a greater degree of anion redox. We understand this stabilization by considering the thermodynamically favored states of the oxidized material which would be pyrite FeS2 in the case of Li2FeS2. Pyrite FeS2 contains all Fe2+ and persulfides, (S2)2-, indicating that S2- can reduce Fe3+. Indeed, if we anneal Li2FeS2 in the charged state (which contains some Fe3+), we observe FeS2 formation. The Al substitution, however, suppresses this phase transition thereby enabling greater degrees of oxidation.
54. “Transport Characterization of Solid-State Li2FeS2 Cathodes From a Porous Electrode Theory Perspective”
Tim Bernges*, Lukas Ketter, Bianca Helm, Marvin A. Kraft, Kimberly A. See, Wolfgang Zeier*
EES Batteries, 2025, 1, 172-184.
https://dx.doi.org/10.1039/D4EB00005F
53. “Modular MPS3-Based Frameworks for Superionic Conduction of Monovalent and Multivalent Ions”
Zachery W. B. Iton, Zion Irving-Singh, Son-Jong Hwang, Amit Bhattacharya, Sammy Shaker, Tridip Das, Raphaële J. Clément, William A. Goddard III, Kimberly A. See*.
J. Am. Chem. Soc. 2024, 146, 24398–24414.
tl;dr The high charge density of multivalent ions makes it difficult for those ions to conduct in solids. We show that screening that charge with solvent molecules, like water, within a functionally solid material can yield materials with very high conductivity. The strategy is translatable to many different ions. Within the host material, the multivalent ions can hop between solvent-coordinated sites. Further, the water can be swapped out for aprotic solvents which expands their utility. The tunability of these materials allows for the explicit exploration of many structure-property relationships.
52. “Alkali-Independent Anion Redox in LiNaFeS2”
Michelle D. Qian, Eshaan S. Patheria, Nicholas V. Dulock, Colin T. Morrell, Kimberly A. See*.
Chem. Mater. 2024, 36, 7953–7966.
tl;dr LiNaFeS2 is known to undergo anion redox in a Li cell and we demonstrate that the same redox processes occur in a Na cell. Despite the similarity in charge compensation processes, cycling in a Na cell is accompanied by changes in electrochemical behavior including sluggish kinetics. When cycled in a Na cell, the material suffers from particle cracking and loss of crystallinity This work highlights the importance of mitigating structural degradation in the design of high capacity anion redox Na cathodes.
51. “Cation Vacancies Enable Anion Redox in Li Cathodes”
Seong Shik Kim, Daniil A. Kitchaev, Eshaan S. Patheria, Colin T. Morrell, Michelle D. Qian, Jessica L. Andrews, Qizhang Yan, Shu-Ting Ko, Jian Luo, Brent C. Melot, Anton Van der Ven, Kimberly A. See*.
J. Am. Chem. Soc. 2024, 146, 20951–20962.
tl;dr Sulfides can be electrochemically oxidized to form persulfide bonds. The oxidation of the sulfide requires the presence of cation vacancies. Cation vacancies both free up S nonbonding orbitals and allow for the structural distortion required for S-S bond formation. The correlation between cation vacancy and anion oxidation is shown by evaluating two materials with and without vacancies but with the same structure and no available transition metal redox.
50. “The Effect of Metal d Band Position on Anion Redox in Alkali-Rich Sulfides”
Seong Shik Kim, David N. Agyeman-Budu, Joshua J. Zak, Jessica L. Andrews, Jonathan Li, Brent C. Melot, Johanna Nelson Weker, Kimberly A. See*.
Chem. Mater. 2024, 36, 6454-6463.
tl;dr Anion redox is a promising mechanism for next-generation battery cathodes. Sulfides are particularly interesting since sulfides can reversibly oxidize to form persulfides but the voltage is low. We develop to isostructural materials with different transition metals that have different d band levels and show that the voltage of the sulfide oxidation cannot be changed by modulation of the transition metal d band. Thus, sulfide oxidation must be occurring from S nonbonding bands that have little to no metal d character.
49. “Investigating Capacity Fade Mechanisms in Mg-MClx Batteries”
Steven H. Stradley, John-Paul Jones, Ratnakumar V. Bugga, Kimberly A. See*.
J. Electrochem. Soc. 2024, 171, 060501.
tl;dr Metal chlorides are excellent candidate cathodes to pair with a Mg anode due to the beneficial effect of Cl- on Mg electrochemistry. We demonstrate somewhat reversible conversion of metal chlorides based on Earth-abundant metals such as Cu and Fe. However, the cathodes experience dramatic capacity loss with cycling. Chemical reduction by the electrolyte and active material dissolution are identified as the primary causes of capacity fade. Electrolyte modifications can be made to alleviating these factors, laying ground for Mg|MClx chemistries based on abundant materials and with good capacity retention.
48. “Reducing Voltage Hysteresis in Li-Rich Sulfide Cathodes by Incorporation of Mn”
Xiaotong Li, Seong Shik Kim, Michelle D. Qian, Eshaan S. Patheria, Jessica L. Andrews, Colin T. Morrell, Brent C. Melot, Kimberly A. See*.
Chem. Mater. 2024, 36, 5687-5697.
https://doi.org/10.1021/acs.chemmater.4c00736
tl;dr Anion redox is often associated with large voltage hysteresis. In sulfides, anion oxidation causes the formation of S-S bonds which then must be broken upon reduction which is thought to contribute to the voltage hysteresis. Here, we show that incorporation of Mn into the parent phase Li2TiS3 significantly reduces voltage hysteresis. Importantly, the mechanism of sulfide oxidation remains unchanged -- S-S bonds are formed in the oxidized material. Thus, this work shows that S-S bond breaking and forming reactions do not necessarily incur large voltage hysteresis.
47. “A Guide to Troubleshooting Metal Sacrificial Anodes for Organic Electrosynthesis”
Skyler D. Ware, Wendy Zhang, Weiyang Guan, Song Lin, Kimberly A. See*,
Chem. Sci. 2024, 15, 5814-5831.
https://doi.org/10.1039/D3SC06885D
tl;dr Sacrificial anodes enable reductive electrosynthesis by charge-balancing the reaction of interest occurring at the cathode. While sacrificial metal oxidation is often assumed to be straightforward and innocent relative to the chemistry at the cathode, in reality non-ideal processes at the anode can interfere with the reductive reaction. In this perspective, we highlight several common challenges that arise when using sacrificial anodes, and we propose experiments to diagnose and troubleshoot each issue. We anticipate that a thorough understanding of sacrificial anode chemistry will streamline reaction optimization and expand the chemical space available for organic electrosynthesis.
46. “A Mg-In Alloy Interphase for Mg Dendrite Suppression”
Brian C. Lee, Kimberly A. See*,
J. Electrochem. Soc. 2024, 171, 010513.
https://doi.org/10.1149/1945-7111/ad1c13
tl;dr Mg metal batteries have attracted much attention as an alternative to Li-ion technology due to the high abundance and volumetric capacity of Mg metal. Further, early reports show that Mg is less prone to dendritic growth compared to Li, thereby improving the safety and long-term reversibility of Mg metal anodes. However, dendritic growth of Mg can be observed in various conditions, causing cell shorting and capacity loss. Herein, we report a chemically-formed Mg-In alloy interphase that suppresses nonuniform Mg growth during electrochemical reduction. Mg first alloys with the interphase and then deposits underneath as Mg metal at the Mg|Mg-In interface. We find that the kinetics of Mg2+ mobility in the alloy interphase is a key limiting factor for the overall kinetics of the electroreduction.
45. “Enabling Al Sacrificial Anodes in Tetrahydrofuran Electrolytes for Reductive Electrosynthesis”
Wendy Zhang, Weiyang Guan, Yi Wang, Song Lin, Kimberly A. See*,
Chem. Sci. 2023, 14, 13108-13118.
https://dx.doi.org/10.1039/D3SC04725C
tl;dr Al is widely used as a sacrificial anode in organic electrosynthesis. However, the use of an Al sacrificial anode in THF-based electrolytes is commonly accompanied with extremely high cell potentials. In this study, we characterize the Al metal interfaces and manipulate them to improve the performance of an Al sacrificial anode. We have discovered that the presence of halide ions in the electrolyte can yield efficient Al stripping. By incorporating halide additive, we achieve bulk Al stripping in THF-based electrolytes and successfully improve the cell potentials of electrochemically driven reductive methodologies.
44. “Three-Component Cross-Electrophile Coupling: Regioselective Electrochemical Dialkylation of Alkenes”
Lingxiang Wang, Yi Wang, Wendy Zhang, Wen Zhang, Kimberly A. See, Song Lin*,
J. Am. Chem. Soc. 2023, 145, 22298-22304.
https://doi.org/10.1021/jacs.3c06794
43. “Improving the Mg Sacrificial Anode in Tetrahydrofuran for Synthetic Electrochemistry by Tailoring Electrolyte Composition”
Wendy Zhang, Chaoxuan Gu, Yi Wang, Skyler D. Ware, Lingxiang Lu, Song Lin, Yue Qi, Kimberly A. See*,
JACS Au 2023, 3, 2280-2290.
https://doi.org/10.1021/jacsau.3c00305
tl;dr Mg is commonly used as a sacrificial anode in reductive electrosynthesis. While numerous methodologies using a Mg sacrificial anode have been successfully developed, the optimization of Mg stripping in these systems remains empirical. In this study, we seek to understand and manipulate the Mg metal interfaces for a more effective counter electrode in THF-based electrolyte. Our results suggest that electrical double layer, Mg SEI composition, and organic substrates can all impact the performance of a Mg sacrificial anode. By using supporting electrolytes with weakly coordinating cations, or adding halide salt to the electrolyte, we can improve the Mg stripping process and enable a streamlined optimization process for the development of new electrosynthetic methodologies.
42. “Electrochemical Preparation of Sm(II) Reagent Facilitated by Weakly Coordinating Anions”
Skyler D. Ware, Wendy Zhang, David J. Charboneau, Channing K. Klein, Sarah E. Reisman, and Kimberly A. See*,
Chem. Eur. J. 2023, 29, e202301045.
https://doi.org/10.1002/chem.202301045
tl;dr SmI2 is widely used in organic synthesis as a 1e- reducing agent, but its application in chemically catalytic systems is hindered by poor mechanistic understanding and the need for sacrificial reductants. We investigate the electrochemical reduction of Sm(III) to Sm(II) in pursuit of a Sm-electrocatalytic system. We show that weakly coordinating anions promote Sm redox, and the anion coordination strength can be modulated through ion exchange with the supporting electrolyte. We further demonstrate electrochemical preparation of SmI2 at bulk scale and show that electrogenerated SmI2 performs similarly to commercial SmI2 solutions in a proof of concept organic reaction.
41. “Water Vapor Induced Superionic Conductivity in ZnPS3”
Zachery W. B. Iton, Brian C. Lee, Abigail Y. Jiang, Seong Shik Kim, Michael J. Brady, Sammy Shaker, and Kimberly A. See*,
J. Am. Chem. Soc. 2023, 145, 13312-13325.
https://doi.org/10.1021/jacs.3c03368
tl;dr Development of batteries based on multivalent working ions is hindered by the lack of understanding of multivalent ionics in solids. ZnPS3 has been previously shown to conduct Zn2+ with low activation energy, but with low conductivity. Exposure of ZnPS3 to humidity results in water adsorption in the grain boundaries, unlocking superionic conductivity. The conductivity is due to both superionic H+ conduction and superionic Zn2+ conduction. Zn2+ conducts by solvating from ZnPS3 into the water layer in the grain boundaries, leaving it's anion behind in the solid.
40. “Identification of Potential Solid-State Li-Ion Conductors with Semi-Supervised Learning”
Forrest A. L. Laskowski,† Daniel B. McHaffie,† and Kimberly A. See*,
Energy Environ. Sci. 2023, 16, 1264-1276 († contributed equally).
https://doi.org/10.1039/D2EE03499A
tl;dr The discovery of novel solid-state electrolytes (SSEs) is necessary to meet the conductivity and stability requirements of all-solid-state batteries. We develop a semi-supervised approach for identifying potentially highly conductive SSEs and screen over 26,000 Li-containing materials. Through additional bond-valence site energy and nudged-elastic band calculations, Li3BS3 is identified as a promising compound for experimental validation. We demonstrate that with defects engineered through aliovalent substitution and high-energy ball milling, Li3BS3 exhibits a room-temperature conductivity greater than 1 mS cm-1.
39. “Effect of Polysulfide Speciation on Mg Anode Passivation in Mg-S batteries”
Michelle D. Qian, Forrest A. L. Laskowski, Skyler D. Ware, and Kimberly A. See*,
ACS Appl. Mater. Interfaces 2023, 15, 9193-9202.
http://dx.doi.org/10.1021/acsami.2c19488
tl;dr Mg-S batteries use a Mg metal electrode and a S8 cathode. Electrochemical reduction of S8 during discharge forms intermediate species called polysulfides, [Sx]2-, that are soluble in the electrolyte. The soluble polysulfides can diffuse through the electrolyte and react with the Mg metal electrode. We probe the reactivity of polysulfides based on their chain lengths. Shorter chain polysulfides (small x) lead to rapid reaction at Mg causing electrode passivation, and the passivating layer was determined via XPS to consist of MgSx and electrolyte decomposition products. Longer chain polysulfides react slower, and the equilibrium of short to long can be shifted by adding elemental S8. Addition of S8 to short chain polysulfide solutions reduces the passivation effects on Mg.
38. “Irreversible Anion Oxidation Leads to Dynamic Charge Compensation in Ru-Poor, Li-Rich Cathode Li2Ru0.3Mn0.7O3”
Joshua J. Zak, Mateusz Zuba, Zachary W. Lebens-Higgins, Heran Huang, Matthew J. Crafton, Nathan F. Dalleska, Bryan D. McCloskey, Louis F. J. Piper, and Kimberly A. See*,
ACS Energy Lett. 2023, 8, 722-730.
tl;dr Li-rich metal oxides that contain >1 mol equivalent Li per transition metal have higher theoretical capacities compared to conventional cathodes with one Li per transition metal. We show using several complimentary characterization techniques that upon oxidation of a Li-rich oxide Li2Ru0.3Mn0.7O3, the charge compensation can be attributed a bit to Ru but mostly to side reactions including O2 evolution. We compare bulk and surface characterization to better understand the side reactions. Upon reduction, Ru is reduced past its oxidation state in the pristine material thereby activating it for subsequent reduction. Mn becomes increasingly active as the cell cycles, leading to a material that essentially cycles on the transition metals, just like conventional materials.
37. “An Exploration of Sulfur Redox in Lithium Battery Cathodes”
Joshua J. Zak, Seong Shik Kim, Forrest A. L. Laskowski, and Kimberly A. See*,
J. Am. Chem. Soc. 2022, 144, 10119-10132.
tl;dr Although the d electrons of transition metals are easily accessed to provide the charge compensation required for (d)lithiation reactions (think the redox couples that are well-known in the d block), anions can also contribute to increase capacity. We discuss the ability of the S 3p electrons to participate in the charge compensation. The consequent structural changes range from minimal, i.e. intercalation chemistry, to massive, i.e. phase conversion, and depend on the degree to which S participates. We specifically highlight a hybrid mechanism that undergoes intermediate structural changes and incorporates desirable elements of both intercalation and conversion. A survey of materials with thermodynamically stable persulfides is also reported to provide a starting point for discovery of new materials with stable S redox.
36. “Promoting Reversibility of Multielectron Redox in Alkali-Rich Sulfide Cathodes through Cryomilling”
Seong Shik Kim, David N. Agyeman-Budu, Joshua J. Zak, Andrew Dawson, Qizhang Yan, Miguel Cában-Acevedo, Kamila M. Wiaderek, Andrey A. Yakovenko, Yiyi Yao, Ahamed Irshad, Sri R. Narayan, Jian Luo, Johanna Nelson Weker, Sarah H. Tolbert, and Kimberly A. See*,
Chem. Mater. 2022, 34, 3236-3245.
tl;dr LiNaFeS2 is an alkali-rich metal sulfide that exhibits reversible multielectron redox (> 1.5 electrons), but experiences more rapid capacity fade compared to isostructural Li2FeS2. With transmission X-ray microscopy, we identify the source of capacity fade in LiNaFeS2 to be strain-induced particle fracturing associated with Na+ removal during charge. Particle fracturing in LiNaFeS2 is prevented by cryomilling the material, which reduces the crystallite size and increases microstrain within the particle. We demonstrate that the introduction of defects enables the particle to accommodate strain associated with removal/insertion of large metal ions.
35. “Metal-Metal Bonding as an Electrode Design Principle in the Low-Strain Cluster Compound LiScMo3O8”
Kira E. Wyckoff, Jonas L. Kaufman, Sun Woong Baek, Christian Dolle, Joshua J. Zak, Jadon Bienz, Linus Kautzsch, Rebecca C. Vincent, Arava Zohar, Kimberly A. See, Yolita M. Eggeler, Laurent Pilon, Anton Van der Ven*, and Ram Seshadri*,
J. Am. Chem. Soc. 2022, 144, 5841-5854.
34. “Electrochemically Driven Cross-Electrophile Coupling of Alkyl Halides”
Wen Zhang, Lingxiang Lu, Wendy Zhang, Yi Wang, Skyler D. Ware, Jose Mondragon, Jonas Rein, Neil Strotman, Dan Lehnherr,
Kimberly A. See*, and Song Lin*,
Nature 2022, 604, 292-297.
tl;dr The selective construction of C(sp3)–C(sp3) bonds has been a critical challenge in modern synthetic organic chemistry. In this work with the Lin group, electroreduction of a more substituted alkyl halide is selectively activated to form a carbanion, which undergoes preferential coupling with a less substituted alkyl halide to afford the desired C–C bond via SN2 mechanism. Gram-scale reactions are realized through addition of DME as a co-solvent to stabilize the sacrificial Mg anode. The transition-metal free electrochemically driven cross-electrophile coupling (e-XEC) exhibits high chemoselectivity and broad functional group compatibility versus existing methodologies.
32. “Multivalent Ion Conduction in Inorganic Solids”
Zachery W. B. Iton and Kimberly A. See*,
Chem. Mater, 2022, 34, 881-898.
33. “Hysteresis in Electrochemical Systems”
Anton Van der Ven*, Kimberly A. See, and Laurent Pilon,
Battery Energy 2022, 1, 20210017.
https://doi.org/10.1002/bte2.20210017
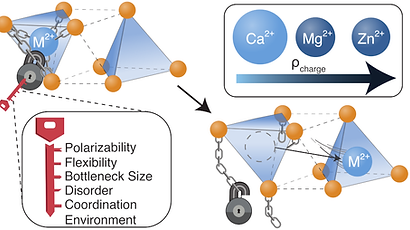
tl;dr Multivalent (MV) ion conduction in inorganic solids is difficult to achieve due to the strong Coulombic interactions that result from the high charge density of MV ions. This perspective discusses how a solid state MV ion conductor can be identified or developed by considering screening of the MV ion charge, the geometry of the conduction pathway, the polarizability of the anion sublattice the coordination environment of the mobile ion and the structural flexibility of components of the material.
31. “Mg Anode Passivation Caused by Reaction of Dissolved Sulfur in Mg-S Batteries”
Forrest A. L. Laskowski, Steven H. Stradley, Michelle D. Qian, and Kimberly A. See*,
ACS Appl. Mater. Interfaces, 2021, 13, 29461-29470.
tl;dr Polysulfide introduction into the magnesium aluminum chloride complex (MACC) electrolyte impedes Mg2+ reduction on Mg anodes. Electrochemical experiments reveal that large reduction overpotentials arise due to the formation of a passivation layer, presumably MgS, on the anode surface. Interestingly, overpotentials are inversely correlated with the concentration of added S8, suggesting that polysulfide disproportion equilibria mediate the extent of anode passivation
30. “Fluoride in the SEI Stabilizes the Li Metal Interface in Li-S Batteries with Solvate Electrolytes”
Skyler D. Ware, Charles J. Hansen, John-Paul Jones, John Hennessy, Ratnakumar V. Bugga, and Kimberly A. See*,
ACS Appl. Mater. Interfaces 2021 13, 18865-18875.
tl;dr Highly concentrated solvate electrolytes are interesting candidates for Li-S batteries as they show low solubility of intermediate lithium polysulfides. We show that the solvate electrolyte in acetonitrile is not stable vs. Li metal at elevated temperatures. Addition of a fluoroether to the electrolyte causes more fluoride in the solid electrolyte interphase (SEI) on Li, reducing SEI impedance and improving surface stability. An additional AlF3 coating on the Li metal further minimizes electrolyte decomposition, and improves cyclability in Li-S cells
29. “From Solid Electrolyte to Zinc Cathode: Vanadium Substitution in ZnPS3”
Andrew J. Martinolich, Skyler D. Ware, Brian C. Lee, and Kimberly A. See*,
J. Phys. Mater. 2021, 4, 024005.
tl;dr Aliovalent substitution of vanadium for zinc in the divalent ion conducting lattice of ZnPS3 imparts reversible electrochemical energy storage of Zn2+ cations in nonaqueous electrolytes, enabled by V centered redox. The aliovalent cation substitution introduces vacancies on the metal sites, making it possible for the materials to be either oxidized or reduced from the pristine state. While the capacity of the materials is observed to increase with increasing V content, it is limited to filling half of the introduced vacancies, correlating to the reduction of half of the V centers. We hypothesize that the limitation is due to changes in the electronic structure of the materials upon V reduction, despite the presence of more vacancies on an ionically conducting lattice that would allow greater capacity. The results highlight the importance of electronic and physical structural characteristics in the design of cathode materials for next generation batteries using divalent working ions.
28. “Controlling Covalency and Anion Redox Potentials through Anion Substitution in Li-Rich Chalcogenides”
Andrew J. Martinolich,† Joshua J. Zak,† David N. Agyeman-Budu, Seong Shik Kim, Nicholas H. Bashian, Ahamed Irshad, Sri R. Narayan, Brent C. Melot, Johanna Nelson Weker, and Kimberly A. See*,
Chem. Mater. 2021, 33, 378-391 († contributed equally).
tl;dr Materials across the solid solution, Li2FeS2-ySey, exhibit reversible multielectron (≥1.5 electrons) redox enabled by mixed cation and anion redox. Substituting Se for S increases the metal-chalcogenide covalency and systematically tunes the anion oxidation potential. By X-ray absorption spectroscopy, Fe, S, and Se oxidation occur throughout charging contrary to discrete cation and anion oxidation observed in Li2FeS2. Introduction of Se results in Se–Se dimers but also the irreversible formation of a new, high-impedance phase at early states of charge, leading to poor cyclability. As such, we assert that while metal-chalcogenide covalency provides a handle to control the voltage of anion oxidation, it is necessary to consider structural changes incurred to design next-generation multielectron storage materials.
27. “Activating Mg Electrolytes through Chemical Generation of Free Chloride and Removal of Trace Water”
Seong Shik Kim and Kimberly A. See*,
ACS Appl. Mater. Interfaces 2021, 13, 671-680.
tl;dr The speciation of the magnesium aluminum chloride complex (MACC) electrolyte upon the addition of a low concentration of Mg(HMDS)2 is explored via Raman spectroscopy, 27Al NMR spectroscopy, and 1H-29Si HMBC. Changes in bulk speciation suggest two roles of Mg(HMDS)2 in MACC: (1) Mg(HMDS)2 scavenges trace water in solution and (2) Mg(HMDS)2 reacts with AlCl4- to form free chloride. The free chloride is actively involved at the interface in facilitating magnesium electrodeposition and stripping.
26. “Selective Formation of Pyridinic-Type Nitrogen-doped Graphene and Its Application in Lithium-Ion Battery Anodes”
Jacob D. Bagley, Deepan Kishore Kumar, Kimberly A. See, and Nai-Chang Yeh*,
RSC Advances 2020, 10, 39562-39571.
https://doi.org/10.1039/D0RA06199A
25. "A Super-Oxidized Radical Cationic Icosahedral Boron Cluster"
Julia M. Stauber, Josef Schwan, Xinglong Zhang, Jonathan C. Axtell, Dahee Jung, Brendon J. McNicholas, Paul H. Oyala, Andrew J. Martinolich, Jay R. Winkler, Kimberly A. See, Thomas F. Miller III*, Harry B. Gray*, and Alexander M. Spokoyny*,
J. Am. Chem. Soc. 2020, 142, 12948-12953.
https://doi.org/10.1021/jacs.0c06159
24. "Understanding the Role of Crystallographic Shear on the Electrochemical Behavior of Niobium Oxyfluorides"
Nicholas H. Bashian, Molleigh B. Preefer, JoAnna Milam-Guerrero, Joshua J. Zak, Charlotte Sendi, Suha Ahsan, Rebecca Vincent, Ralf Haiges, Kimberly A. See, Ram Seshadri, and Brent C. Melot*,
J. Mat. Chem. A 2020, 8, 12623-12632.
https://doi.org/10.1039/D0TA01406K
23. "Multielectron, Cation and Anion Redox in Lithium-Rich Iron Sulfide Cathodes"
Charles J. Hansen,† Joshua J. Zak,† Andrew J. Martinolich, Jesse S. Ko, Nicholas H. Bashian, Farnaz Kaboudvand, Anton Van der Ven,
Brent C. Melot, Johanna Nelson Weker, and Kimberly A. See*,
J. Am. Chem. Soc. 2020, 142, 6737-3749 († contributed equally).
tl;dr Two isostructural alkali-rich metal sulfides - Li2FeS2 and previously unreported LiNaFeS2 - demonstrate reversible multielectron redox (>= 1.5 electrons). We probe the charge storage mechanism and find that both cationic and anionic redox contribute. In the beginning of the charge profile, the materials undergo a deintercalation-like mechanism in which Fe2+ is oxidized to Fe2+/3+. Oxidation of Fe2+ causes the Fe and S bands to rehybridize, increasing the covalency of the Fe-S correlations and pushing the S 2p states closer to the Fermi level. Subsequent oxidation occurs on the anions, (S)2-, to form (S2)2- moieties causing loss of long-range order. The anion oxidation is clearly observed in S K-edge X-ray absorption spectroscopy.
22. "Conditioning-Free Electrolyte by Minor Addition of Mg(HMDS)2"
Seong Shik Kim, Sarah C. Bevilacqua, and Kimberly A. See*,
ACS Appl. Mater. Interfaces 2020, 12, 5226-5233.
tl;dr Addition of small concentrations of Mg(HMDS)2 reduces cathodic current associated with Al deposition in the magnesium aluminum chloride complex (MACC) electrolyte, resulting in a conditioned electrolyte on cycle 1. Such a drastic change in the electrochemistry from addition of a very small concentration of Mg(HMDS)2 suggests that the effect is localized to the electrode-electrolyte interface. Electrochemical experiments suggest that addition of Mg(HMDS)2 not only scavenges water, but also causes a secondary effect that we hypothesize is the formation of free Cl-.
21. "Dense Garnet-Type Electrolyte with Coarse Grains for Improved Air Stability and Ionic Conductivity"
Xiaomei Zeng, Andrew J. Martinolich, Kimberly A. See, and Katherine T. Faber*,
J. Energy Storage 2020, 27, 101128.
https://doi.org/10.1016/j.est.2019.101128
20. "Effect of the Electrolyte Solvent on Redox Processes in Mg-S Batteries"
Sarah C. Bevilacqua, Kim H. Pham, and Kimberly A. See*,
Inorg. Chem. 2019, 58, 10472-10482.
tl;dr Mg deposition and stripping is demonstrated with a MgCl2 and AlCl3 electrolyte in a variety of ethereal solvents. The new electrolyte systems are used to systematically study solvent effects on sulfur electroreduction in Mg-S cells. Irreversible sulfur reduction is observed when Mg-S cells are prepared with the electrolytes, and the peak potential is found to vary with solvent suggesting that the electrolyte is active in the reduction mechanism.
19. "Solid State Divalent Ion Conductivity in ZnPS3"
Andrew J. Martinolich, Cheng-Wei Lee, I-Te Lu, Sarah C. Bevilacqua, Molleigh B. Preefer, Marco Bernardi, André Schleife*, and
Kimberly A. See*,
Chem. Mater. 2019, 31, 3652-3661.
http://dx.doi.org/10.1021/acs.chemmater.9b00207
tl;dr ZnPS3 supports divalent ion conduction, Zn2+, with low activation energies (350 meV). Zn2+ diffuses via a vacancy-mediated mechanism within the metal layer. The transition state that defines the activation energy along the Zn2+ diffusion pathway involves an extension of the P-P-S bond angle in [P2S6]4-, pushing S into the van der Waals gap.
prior to Caltech
18. “Elucidating Zn and Mg Electrodeposition Mechanisms in Nonaqueous Electrolytes for Next-Generation Metal Batteries”
Kim Ta, Kimberly A. See, and Andrew A. Gewirth,
J. Phys. Chem. C 2018, 122, 13790-13796.
http://dx.doi.org/10.1021/acs.jpcc.8b00835
17. “The Effect of the Hydrofluoroether Cosolvent Structure in Acetonitrile-based Solvate Electrolytes on Li+ Solvation Structure and Li‒S Battery Performance”
Minjeong Shin, Heng-Liang Wu, Badri Narayanan, Kimberly A. See, Rajeev S. Assary, Lingyang Zhu, Richard T. Haasch, Shuo Zhang, Zhengcheng Zhang, Larry A. Curtiss, and Andrew A. Gewirth,
ACS Appl. Mater. Interfaces. 2017, 9, 39357-39370.
http://dx.doi.org/10.1021/acsami.7b11566
16. “Effect of Concentration on the Electrochemistry and Speciation of the Magnesium Aluminum Chloride Complex Electrolyte Solution”
Kimberly A. See, Yao-Min Liu, Yeyoung Ha, Christopher J. Barile, and Andrew A. Gewirth,
ACS Appl. Mater. Interfaces. 2017, 9, 35729-35739.
http://dx.doi.org/10.1021/acsami.7b08088
15. “Reversible Capacity of Carbon Additives at Low Potentials: Caveats for Testing Alternative Anode Materials,”
Kimberly A. See, Margaret A. Lumley, Galen D. Stucky, Clare P. Grey, and Ram Seshadri,
J. Electrochem. Soc. 2017, 164, A327-A333.
http://dx.doi.org/10.1149/2.0971702jes
14. “Thiol-Based Electrolyte Additives for High-Performance Lithium-Sulfur Batteries”
Heng-Liang Wu, Minjeong Shin, Yao-Min Liu, Kimberly A. See, and Andrew A. Gewirth,
Nano Energy. 2017, 32, 50-58.
http://dx.doi.org/10.1016/j.nanoen.2016.12.015
13. “Effect of Hydrofluoroether Cosolvent Addition on Li Solvation in Acetonitrile-Based Solvate Electrolytes and Its Influence on S Reduction in a Li-S Battery”
Kimberly A. See, Heng-Liang Wu, Kah Chun Lau, Mingjeong Shin, Lei Cheng, Mahalingam Balasubramanian, Kevin G. Gallagher, Larry A. Curtiss, and Andrew A. Gewirth,
ACS Appl. Mater. Interfaces. 2016, 8, 34360-34371.
http://dx.doi.org/10.1021/acsami.6b11358
12. “Practical Stability Limits of Magnesium Electrolytes”
Albert L. Lipson, Sang-Don Han, Baofei Pan, Kimberly A. See, Andrew A. Gewirth, Chen Liao, John T. Vaughey, and Brian J. Ingram,
J. Electrochem. Soc. 2016, 163, A2253-A2257.
http://dx.doi.org/10.1149/2.0451610jes
11. “The Interplay of Al and Mg Speciation in Advanced Mg Battery Electrolyte Solutions”
Kimberly A. See, Karena W. Chapman, Lingyang Zhu, Kamila M. Wiaderek, Olaf J. Borkiewicz, Christopher J. Barile, Peter J. Chupas, and Andrew A. Gewirth,
J. Am. Chem. Soc. 2016, 138, 328-337.
http://dx.doi.org/10.1021/jacs.5b10987
10. “Nanostructured Mn-Doped V2O5 Cathode Material Fabricated from Layered Vanadium Jarosite”
Hongmei Zeng, Deyu Liu, Yichi Zhang, Kimberly A. See, Young-Si Jun, Guang Wu, Jeffrey A. Gerbec, Xiulei Ji, and Galen D. Stucky,
Chem. Mater. 2015, 27, 7331–7336.
http://dx.doi.org/10.1021/acs.chemmater.5b02840
9. “Lithium Charge Storage Mechanisms for Cross-Linked Triazine Networks and Their Porous Carbon Derivatives”
Kimberly A. See, Stephan Hug, Katharina Schwinghammer, Margaret A. Lumley, Yonghao Zheng, Jaya M. Nolt, Galen D. Stucky, Fred Wudl, Bettina V. Lotsch, and Ram Seshadri,
Chem. Mater. 2015, 27, 3821-3829.
http://dx.doi.org/10.1021/acs.chemmater.5b00772
8. “X-ray Diffraction Computed Tomography for Structural Analysis of Electrode Materials in Batteries”
Kristin M. Ø. Jensen, Xiaohao Yang, Josefa Vidal Laveda, Wolfgang G. Zeier, Kimberly A. See, Marco D. Michiel, Brent C. Melot, Serena A. Corr, and Simon J. L. Billinge,
J. Electrochem. Soc. 2015, 162, A1310-A1314.
http://dx.doi.org/10.1149/2.0771507jes
7. “Ab initio Structure Search and in situ 7Li NMR Studies of Discharge Products in the Li-S Battery System”
Kimberly A. See, Michal Leskes, John M. Griffin, Sylvia Britto, Peter D. Matthews, Alexandra Emly, Anton Van der Ven, Dominic S. Wright, Andrew J. Morris, Clare P. Grey, and Ram Seshadri,
J. Am. Chem. Soc. 2014, 136, 16368-16377.
http://dx.doi.org/10.1021/ja508982p
6. “A Stable Polyaniline-Benzoquinone-Hydroquinone Supercapacitor”
David Vonlanthen, Pavel Lazarev, Kimberly A. See, Fred Wudl, and Alan J. Heeger,
Adv. Mater. 2014, 26, 5095-5100.
http://dx.doi.org/10.1002/adma.201400966
5. “Sulfur-functionalized Mesoporous Carbons as Sulfur Hosts in Li-S Batteries: Increasing the Affinity of Polysulfide Intermediates to Enhance Performance”
Kimberly A. See, Young-Si Jun, Jeffrey A. Gerbec, Johannes K. Sprafke, Fred Wudl, Galen D. Stucky, and Ram Seshadri,
ACS Appl. Mater. Interfaces. 2014, 6, 10908-10916.
http://dx.doi.org/10.1021/am405025n
4. “Sulfur Infiltrated Mesoporous Graphene-Silica Composite as a Polysulfide Retaining Cathode Material for Lithium-Sulfur Batteries”
Kyoung Hwan Kim, Young-Si Jun, Jeffrey A. Gerbec, Kimberly A. See, Galen D. Stucky, Hee-Tae Jung,
Carbon. 2014, 69, 543-551.
http://dx.doi.org/10.1016/j.carbon.2013.12.065
3. “Bimodal Mesoporous Titanium Nitride/Carbon Microfibers as Efficient and Stable Electrocatalysts for Li-O2 Batteries”
Jihee Park, Young-Si Jun, Woo-ram Lee, Jeffrey A. Gerbec, Kimberly A. See, and Galen D. Stucky,
Chem. Mater. 2013, 25, 3779-3781.
http://dx.doi.org/10.1021/cm401794r
2. “A High Capacity Calcium Primary Cell Based on the Ca-S System"
Kimberly A. See, Jeffrey A. Gerbec, Young-Si Jun, Fred Wudl, Galen D. Stucky, and Ram Seshadri,
Adv. Energy Mater. 2013, 8, 1056-1061.
http://dx.doi.org/10.1002/aenm.201300160
1. “Mesostructured Block Copolymer Nanoparticles: Versatile Templates for Hybrid Inorganic/Organic Nanostructures”
Luke A. Connal, Nathaniel A. Lynd, Maxwell J. Robb, Kimberly A. See, Se Gyu Jang, Jason M. Spruell, and Craig J. Hawker,
Chem. Mater. 2012, 24, 4036-4042.
http://dx.doi.org/10.1021/cm3011524